A new diagnostic technology that quickly identifies viral or bacterial infections by dropping a single drop of blood on artificial vessels-on-a-chip that mimics the human immune response has been developed. In addition to its ability to detect infections before they begin to show symptoms (i.e., fever), the new method is ready to use and does not need a complicated instrument.
A research team, led by Professor Joo H. Kang in the Department of Biomedical Engineering at UNIST has reported a microfluidic device that can detect and identify important bacterial pathogens in their early stages of growth. In their study, the research team designed and fabricated an inflammatory vascular endothelium-mimicking microfluidic channels, coated with adhesion molecules of the endothelium in a combination of P-selectin, E-selectin, and ICAM-1 to distinguish blood samples of septic and healthy individuals. Indeed, when the infected blood samples were injected into the CAMs-coated microfluidic channel, leukocytes became more adherent to the CAMs on inflammatory endothelium compared to the leukocytes in a normal condition.
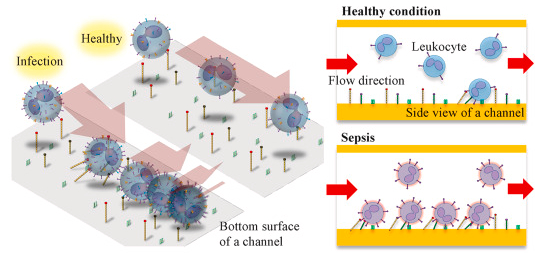 Schematic illustrations showing differential capture of leukocytes on the inflammatory-mimicking microfluidic device for infection diagnosis. (c) A schematic representation of the microfluidic infection stratification based on enumerating the activated leukocytes captured on the CAMs-coated microfluidic channel.
Schematic illustrations showing differential capture of leukocytes on the inflammatory-mimicking microfluidic device for infection diagnosis. (c) A schematic representation of the microfluidic infection stratification based on enumerating the activated leukocytes captured on the CAMs-coated microfluidic channel.
With the help of this device, the time required to take the test is short, lasting less than 10 minutes. Besides, even during the early stages of infection (i.e., the first hours after infection), the detection of latent stage patients that show no signs of infection can be identified early. And thus, this is expected to radically change the method of the current screening and testing for COVID-19 that mainly depends on physical examinations or body temperature assessment.
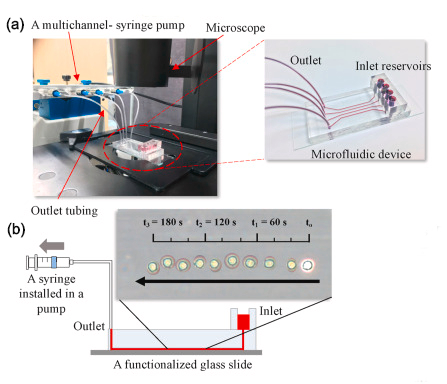
Preparation of the microfluidic device for the detection of infection based on differential leukocyte capture. (a) An experimental setting and the microfluidic device for infection diagnosis. (b) The superimposed images of leukocyte rolling on a CAMs-coated microchannel substrate (time interval: 20s).
The research team imitated the phenomenon, in which the effector cells of the immune system (white blood cells) adhere to the inner surface of the blood vessels (diapedesis), as they move through them to migrate toward the area of virus infection. The inner surfaces of the proposed device are coated with adhesion molecules of the endothelium in a combination of P-selectin, E-selectin, and ICAM-1 to distinguish blood samples of septic and healthy individuals. These proteins facilitate more frequent capture of leukocytes from blood samples sent through these channels. Besides, an increase in protein expression paired with the proteins inside the lining of blood vessels can be achieved on the surfaces of white blood cells in patients, so does the percentage of the captured leukocytes. For this reason, the frequency of leukocytes captured in the CAMs-coated microfluidic channel would increase when flowing the septic blood through the device compared to that of the healthy individuals.
“It has been well known that the expression levels of specific proteins within the internal lining of the blood vessels increases upon infection,” says Research Professor Seyong Kwon in the Department of Biomedical Engineering at UNIST. “Through this research, we have discovered that an increase in protein expression paired with the proteins inside the lining of blood vessels could lead to an increase in the percentage of the captured leukocytes.”
“Since the immune response occurs regardless of the causative bacteria, it can be used to diagnose all kinds of bacteria and viruses, and can be applied to early diagnosis of cancer as well as infectious diseases,” says Amanzhol Kurmashev, the first author of the study.
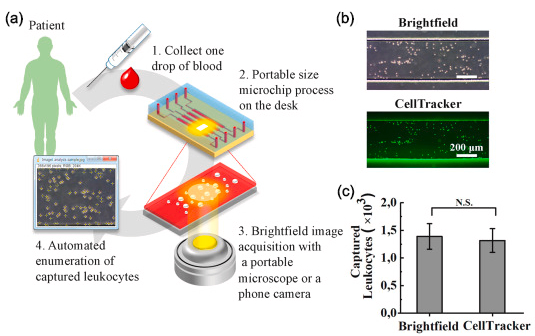
Validation of the differential leukocyte capture for infection stratification inin vivo rat models. (a) A schematic representation of data acquisition with brightfield imaging for potential point-of-care diagnostics. (b) Brightfield and fluorescent CellTracker™ microscopy images of the captured leukocytes. (c) Comparison of the automated cell quantification between the brightfield and fluorescent microscopy images.
The research team leveraged this finding to determine infection by measuring the increased adhesion of leukocytes to an inflammatory vascular endothelium-mimicking microchannel coated with CAMs. They successfully validated that the proposed method can significantly differentiate infection in bacteremia and endotoxemia models in rats as early as an hour post-infection using a finger-prick volume of blood (50 μL), which were unachievable with the conventional diagnostic methods.
“The newly-proposed device delivers fast results when compared to the traditional diagnosis that mainly depends on a blood culture method, even with the supports of state-of-the-art molecular diagnostic tests, such as polymerase chain reaction (PCR),” says Professor Kang. “Besides, the optical microscope used in diagnosis provides a lower magnification, required for the formation of enlarged images, and thus can be mounted on a smartphone.” He adds, “Our ultimate goal is to develop a portable diagnostic system that enables rapid and accurate on-site screening of infectious diseases within 5 to 10 minutes.”
“We believe the robustness of the proposed detection in bright-field imaging mode compels our device increasingly attractive for decentralized diagnostics because it can be integrated into a mobile platform without a costly optical setup,” noted the research team. “Future validation using clinical blood samples with diverse cohorts of patients should be followed to bring the technology to a clinical setting.”
The findings of this research have been published in the online version of Biosensors and Bioelectronics, ahead of print, on August 29, 2020. It has been supported by the grants from the Ministry of Science and ICT (MSIT), the Samsung Research Funding and Incubation Center for Future Technology, and the Ministry of Education.
Journal Reference
Seyong Kwon, Amanzhol Kurmashev, Min Seok Lee, and Joo H. Kang, “An inflammatory vascular endothelium-mimicking microfluidic device to enable leukocyte rolling and adhesion for rapid infection diagnosis,” Biosensors and Bioelectronics, (2020).
