A new study, affiliated with UNIST BME has unveiled the mechanisms involved in the liver metastasis of breast cancer, thanks to the three-dimensional (3D) organ-on-a-chip that mimics human liver functions. This breakthrough is expected to contribute to early detection of metastatic cancer, and thus towards the development and progression for personalized diagnosis and treatment.
A research team, led by Professor Yoon-Kyoung Cho (Department of Biomedical Engineering) and her research team from the Center for Soft and Living Matter (CSLM), within the Institute for Basic Science (IBS) at UNIST have described a 3D microfluidic human liver-on-a-chip (liver-chip) that emulates the formation of a premetastatic niche to investigate the roles of breast cancer-derived extracellular vesicles (EVs) in liver metastasis.
Extracellular vesicles (EVs) are cell-derived membranous vesicles, secreted by virtually most cell types, including cancer cells. Although EVs have recently emerged as intercellular messengers between tumor cells and their microenvironment, a key regulator of tumor progression, the underlying mechanism hsa not been fully elucidated.
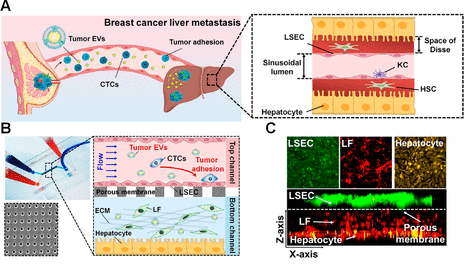
Microfluidic human liver-chip recapitulates breast cancer liver metastasis. (A) Scheme of in vivo breast cancer liver metastasis after premetastatic niche formation by primary tumor-derived EVs (KC: Kupffer cell, HSC: hepatic stellate cell).
In this study, the research team developed an in vitro microfluidic platform that creates a physiologically relevant liver microenvironment in order to investigate how tumor-derived EVs affect liver metastasis. By applying this system, they also demonstrated that breast cancer-derived EVs activate liver sinusoidal endothelial cells (LSECs) in the liver-chip, inducing endothelial to mesenchymal transition and destruction of vessel barriers. In addition, they showed that transforming growth factor β1 (TGFβ1) in breast cancer-derived EVs upregulates fibronectin, an adhesive extracellular matrix protein, on LSECs, which facilitates the adhesion of breast cancer cells to the liver microenvironment.
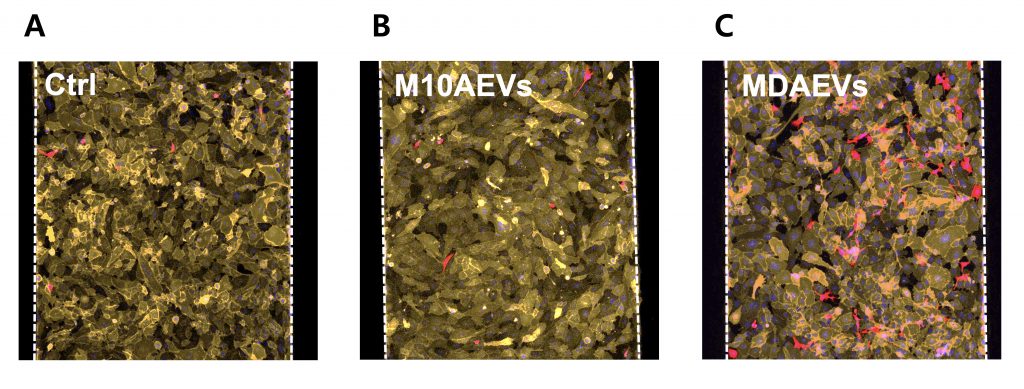
MDAEVs increase MDA cell adhesion to the liver microenvironment in human liver-chips. Effects of various breast epithelial cell-derived EVs on MDA cell adhesion to LSECs in human liver-chips (red: MDA; orange: LSECs; scale bar: 100 μm).
The research team further assessed whether EVs isolated from the blood of breast cancer patients with liver metastases influence the adhesion of tumor cells to the human liver microenvironment emulated by the 3D liver-chip. And they observed that EVs isolated from triple-negative breast cancer (TNBC) patients with liver metastasis contain higher TGFβ1 levels and induce adhesion of more breast cancer cells to the 3D human liver-chip than do EVs isolated from healthy donors or nonmetastatic TNBC patients.
According to the research team, these findings provide a better understanding of the mechanisms through which breast cancer-derived EVs guide secondary metastasis to the liver. They also noted that the 3D human liver-chip described in this study provides a platform to investigate the mechanisms underlying secondary metastasis to the liver and possible therapeutic strategies.
The findings of this research have been published and selected as the front cover of the journal, ACS Nano on November 24, 2020.
“Although we have only assessed the roles of breast cancer cell-derived EVs in the liver metastasis of breast cancer, the engineered human liver-chip can also provide a platform for studying liver metastasis of other types of cancers, such as PDAC and colorectal cancer, which show a high
frequency of secondary metastasis to the liver,” noted Professor Cho. “We, therefore, believe that our device will provide meaningful avenues for studying cancer metastasis and developing therapeutic interventions for secondary metastasis in patients with tumors.”
The research has been carried out in collaboration with Professor Hee Jin Lee and her research team from Asan Medical Center. This research has been supported by the Institute for Basic Science (IBS) and the National Research Foundation of Korea (NRF). The findings of this research have been published and selected as the front cover of the journal, ACS Nano on November 24, 2020.
Journal Reference
Junyoung Kim, Chaeeun Lee, Inun Kim, et al., “Three-Dimensional Human Liver-Chip Emulating Premetastatic Niche Formation by Breast Cancer-Derived Extracellular Vesicles,” ACS Nano, (2020).