The human body is made of numerous different types of cells, which have small compartments known as organelles to perform complex biochemical reactions. These compartments have multiple enzymes that work together to execute important cellular functions. Professor Yoon-Kyoung Cho (Department of Biomedical Engineering) and her research team from the Center for Soft and Living Matter (CSLM), within the Institute for Basic Science (IBS) at UNIST have successfully mimicked these nano spatial compartments to create ‘artificial mitochondria’ in the latest research published in Nature Catalysis. They state the technology can be used to construct artificial organelles that can supply ATP or other useful molecules to cells in damaged or diseased tissues.
This was achieved through reprogramming of ‘exosomes,’ which are small vesicles (diameter ~120 nm) that cells use for intercellular signaling. The researchers carried out the experiments using microfluidic droplet reactors, which generated small droplets that were of similar size as typical cells. (diameter ~10 μm) The researchers first aimed to facilitate controlled fusion of these exosomes within the droplets while preventing unwanted fusions.
They accomplished this by tailoring the exosome surfaces with molecules called catechol, which is a chelating agent that forms complexes with metal ions. This was in turn done by attaching the catechol onto antibodies that target specific cell markers, such as CD9. The complex-forming property of catechol allows them to drive fusions between exosomes when they are mixed with metal ions such as Fe3+. The membrane fusion occurs when the catechols on the surfaces bind to the iron and bring the vesicles to close proximity to one another.
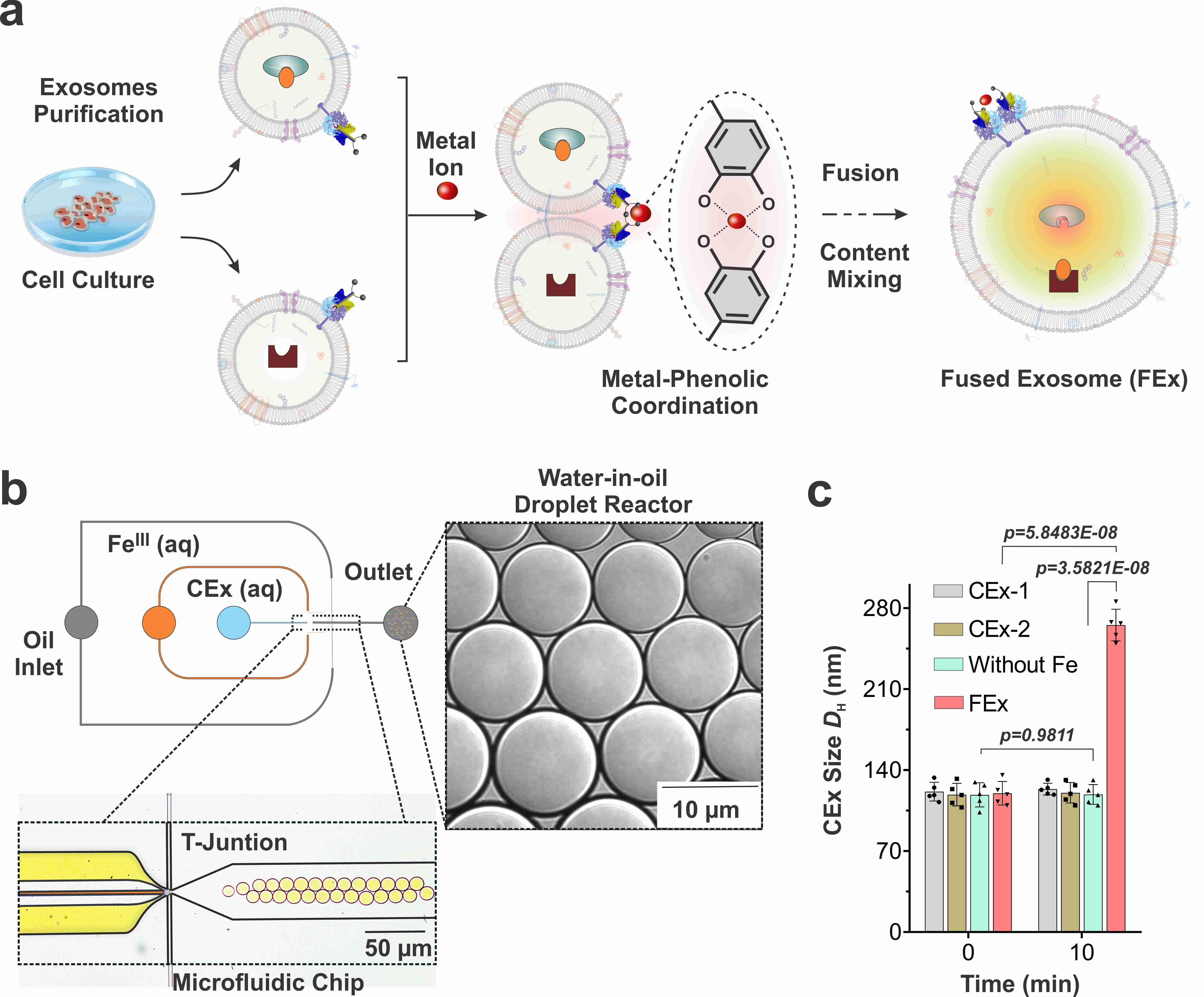
Figure 1. (a) Schematic representation of a two-enzyme GOx/HRP CEx fusing into FEx. The catalytic reaction was initiated by adding glucose, generating H2O2 by aerobic oxidation, which was then used to oxidize Amplex Red to generate resorufin. (b) Representative fluorescence spectra of resorufin generated by biocatalysis in fused exosomes (FEx-GOx-HRP) at different time points. Reaction rate for the nanoreactor vs glucose concentration to calculate kinetic constants by fitting the Michaelis-Menten equation. (c) Stability and integrity of FEx-GOx-HRP after cellular integration after 48 h. Endosomes (EEA1, red) lysosomes (LAMP1, magenta), and FEx (green) are shown. (d) Plot of Pearson correlation coefficient (PCCLysosome-FEx) values over time for magenta (lysosome) and green (FEx) fluorescence, and for red (Endosome) and green (FEx) fluorescence.
Researchers first tested the effectiveness of this system by loading one type of exosomes with calcein-Co2+ and another with EDTA. When the two vesicles fuse and the contents are mixed, EDTA grabs the Co2+ away from calcein, which then allows the latter to fluoresce. The team realized they were successful upon the detection of the fluorescence signal, and the fusion was further confirmed by the doubling of the measured exosome diameter.
These customized exosomes were then preloaded with different reactants and enzymes, which turned them into biomimetic nano factories. This allows them to produce high-value biomolecules by performing desired biocatalytic transformations in a spatially confined manner which is not possible using conventional laboratory test tubes. The team demonstrated this multienzyme biocatalytic cascade function by encapsulating glucose oxidase (GOx) and horseradish peroxidase (HRP) inside the exosomes. The GOx first converts glucose into gluconic acid and hydrogen peroxide. The HRP in turn uses the hydrogen peroxide generated in the first reaction to oxidize Amplex Red to a fluorescent product, resorufin. The researchers were even able to take a step further by adding a third enzyme, galactosidase which converts lactose into glucose, into the mix.
The researchers, then, wanted to know exactly how well these mini reactors can be uptaken and internalized by the cells. The cells derived from human breast tissues were fed with fused exosome nanoreactors, and their internalization over the next 48 hours was observed using various markers and a confocal laser scanning microscope (CLSM). It was found that cells were able to uptake these customized exosomes primarily through endocytosis, along with multiple other mechanisms. They further tested the beforementioned GOx-HRP two enzyme system in the cells, and it was found that the fused exosomes were able to successfully manufacture fluorescent products even while being inside the cells.
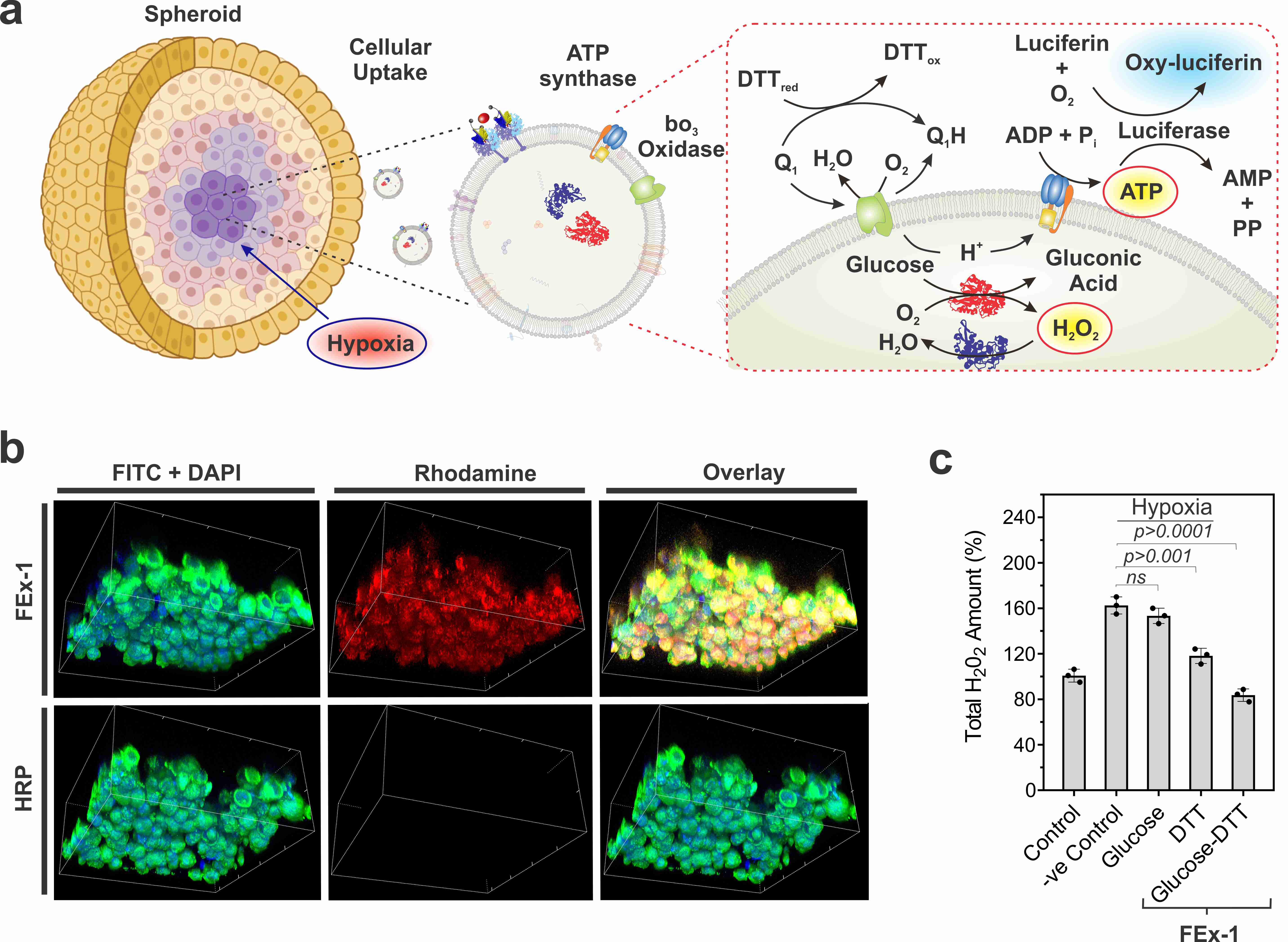 Figure 2. (a) Assembly of artificial organelles for energy generation. ATP synthase was reconstituted into CEx-GOx and bo3 oxidase was reconstituted into CEx-HRP. The two CEx were fused and were able to penetrate deep into spheroids. (b) Decrease in pH (-ΔpH) vs. glucose concentrations for different exosomes. Fused exosomes show a greater degree of pH decrease. (c) Changes in ATP production after FEx-1 uptake under different conditions. (d) Artificial organelles were able to penetrate deep into the spheroid. The top row shows FEx-1 (labeled red with rhodamine B), while the bottom row shows free HRP enzyme inside spheroids. (e) Overall ROS levels decreased on subjecting FEx-1 under glucose and DTT.
Figure 2. (a) Assembly of artificial organelles for energy generation. ATP synthase was reconstituted into CEx-GOx and bo3 oxidase was reconstituted into CEx-HRP. The two CEx were fused and were able to penetrate deep into spheroids. (b) Decrease in pH (-ΔpH) vs. glucose concentrations for different exosomes. Fused exosomes show a greater degree of pH decrease. (c) Changes in ATP production after FEx-1 uptake under different conditions. (d) Artificial organelles were able to penetrate deep into the spheroid. The top row shows FEx-1 (labeled red with rhodamine B), while the bottom row shows free HRP enzyme inside spheroids. (e) Overall ROS levels decreased on subjecting FEx-1 under glucose and DTT.
Armed with this knowledge, the team sought to create functional artificial mitochondria that are capable of producing energy inside the cells. To achieve this, ATP synthase and bo3 oxidase were reconstituted into the earlier exosomes containing GOx and HRP, respectively. These exosomes were in turn fused to create nanoreactors that can produce ATP using glucose and dithiothreitol (DTT). It was found that the fused exosomes were capable of penetrating deep into the core part of a solid spheroid tissue and produce ATP in its hypoxic environment. The activities of these simple organelles were accompanied by marked reduction of pH and reactive oxygen species (ROS) generation. In contrast, free enzymes were unable to penetrate inside these tightly packed spheroids of cells.
“Taken together, our results highlight the potential of these exosomes as nanoreactors in regulating the metabolic activity of cells inside spheroids, and in attenuating cell damage due to hypoxia,” noted Professor Yoon-Kyoung Cho, the corresponding author of the study. It is hoped that further research into such artificial organelles will present a new paradigm in various fields such as disease diagnosis and treatment, biotechnology, medicine, and the environment.
The findings of this research have been published and selected as the front cover of the journal, Nature Catalysis (IF: 41.813) on September 14, 2021.