A recent study, affiliated with UNIST BME has unveiled the mechanism of development of hematopoietic stem cells (HSCs) that give rise to all blood cell types.
In collaboration with the University of California, San Diego (UCSD), Director Kyungjae Myung and Dr. Yoonsung Lee at the Center for Genomic Integrity within the Institute for Basic Science (IBS) at UNIST demonstrated that Supt16h, a component of the Facilitates chromatin transcription (FACT) complex, is required for the formation of hematopoietic stem and progenitor cells (HSPCs).
Blood cells are divided into three main types: the red blood cells (erythrocytes), the white blood cells (leukocytes), and the blood platelets (thrombocytes). Hematopoietic stem cells (HSCs) are multipotent progenitors of blood cells that have self-renewal and proliferation capabilities. Therefore, strict control of the temporal and spatial specification, maturation, and expansion of HSCs is crucial for the normal function of the blood and immune system. It is well known that HSCs arise from the hemogenic endothelium, a special population of endothelial cells within the ventral wall of the dorsal aorta that transdifferentiates into HSCs. In this study, the research team focused on histone chaperone proteins to find a new gene responsible for HSPC formation.
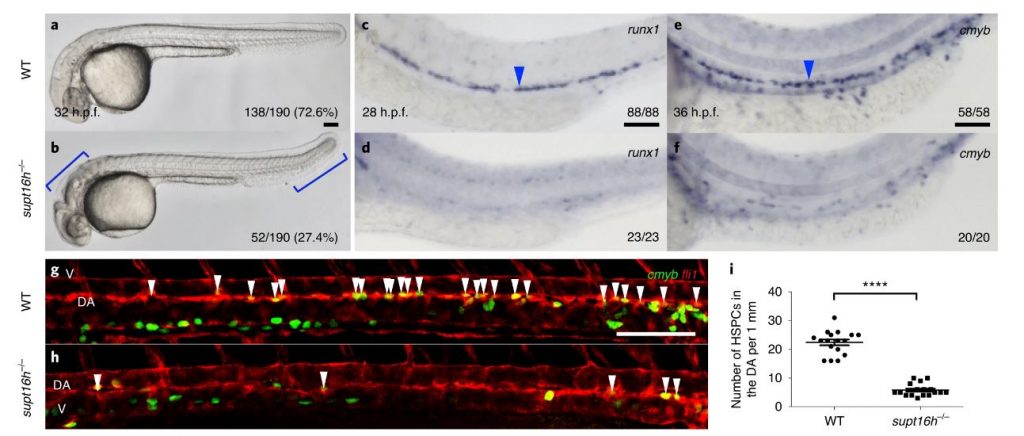
Figure 1: A forward genetic screen identifies supt16h−/− mutants that specifically lack HSPCs.
Histone chaperones are proteins that act as molecular escorts for the prevention of histone-DNA aggregation during nucleosome assembly. They also function as spools for the thread-like DNA to wrap around to prevent it from being tangled. When these proteins are expressed in the wrong place or at the wrong time, it alters cell function and contributes to the progression of cancer and genetic diseases.
Their studies were aimed at understanding how HSCs are specified. We performed a forward genetic screen using zebrafish embryos to find novel genes required for HSC specification, obtaining multiple mutants, which failed to form HSCs in the aorta. “We are currently characterizing the mutant phenotypes and are mapping and positionally cloning genes, which cause the defects of HSC formation. Our studies will provide insight into the molecular cues that specify HSCs,” noted the research team.
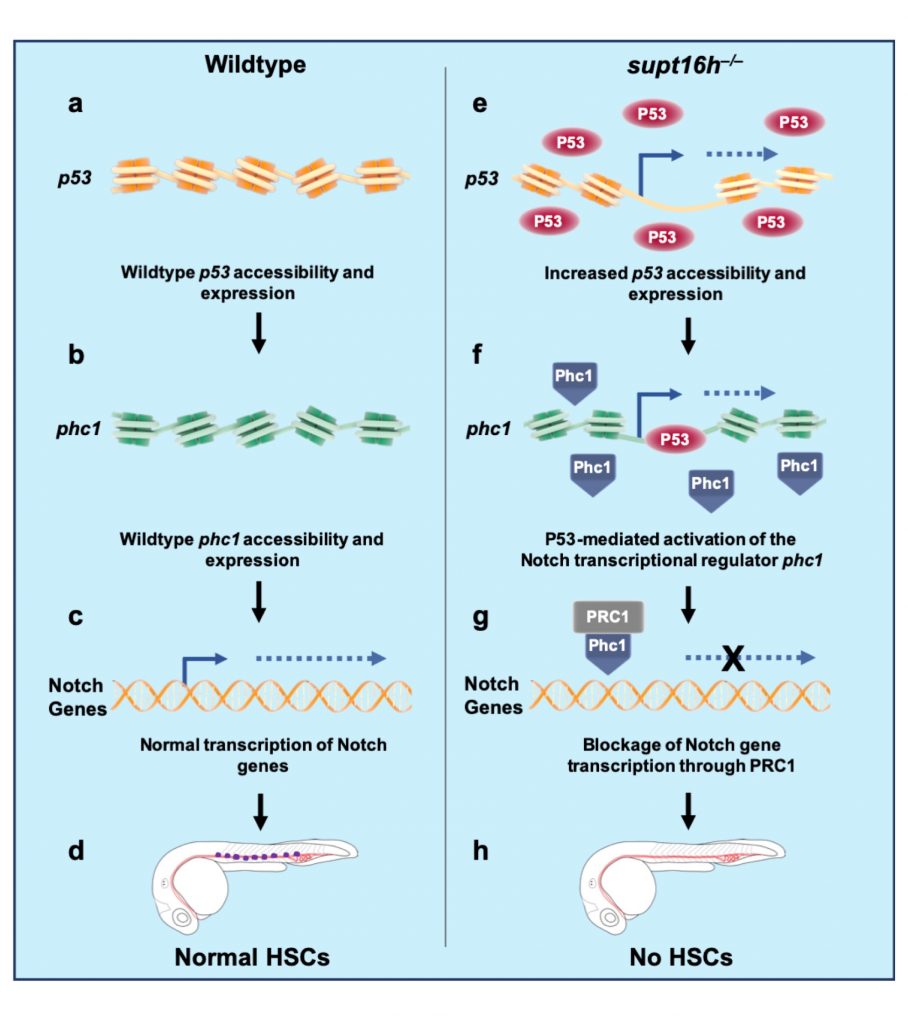
After randomly mutating DNA in 13,000 zebrafish, the research team found that Supt16h plays a key role in developing hematopoietic stem cells via next-generation sequencing analysis. They also observed that zebrafish supt16h mutants express reduced levels of notch-signaling components, inhibiting HSC production.
“The study results have shown that the histone chaperone Supt16h regulates the expression of genes essential for the development of specific cells and that the notch-signaling system plays a key role in HSC production,” said Dr. Lee Yoon-sung at IBS. “We expect our findings to be a significant indicator for improving the treatment of blood diseases, including leukemia and blood cancer, by using HSC.
The findings of this research have been published in the online edition of Nature Cell Biology on November 24, 2020.
Journal Reference
Sophia G. Espanola, Hyemin Song, Eunjin Ryu, et al., “Haematopoietic Stem Cell-Dependent Notch Transcription is Mediated by p53through the Histone Chaperone Supt16h,” Nature Cell Biology, (2020) https://doi.org/10.1038/s41556-020-00604-7.
