
A research team, led by Professors Joo Hun Kang and Jinmyoung Joo in the Department of Biomedical Engineering at UNIST, in collaboration with Professor Jae Hyuk Lee from Seoul National University Bundang Hospital, has introduced a novel technology capable of removing inflammation-triggering agents in extracorporeal blood. The team anticipates that this innovation will open avenues for sepsis treatment by demonstrating excellent therapeutic effects under conditions similar to those of actual patients.
In their study, the team presented a clinically applicable magnetic extracorporeal blood cleansing device utilizing magnetic nanoparticles (MNPs) enveloped by nanovesicles derived from red blood cells (RBC-MNVs). Utilizing superparamagnetic nanoclusters (SPNCs), the new method is capable of rapidly and effectively removing pathogenic and pathogen-related substances that cause sepsis. Their findings from preclinical experiments also validated the clinical utility of their SPNC-based blood cleansing method by treating lethally infected bacteremic model swine that have similar physiological features to humans.
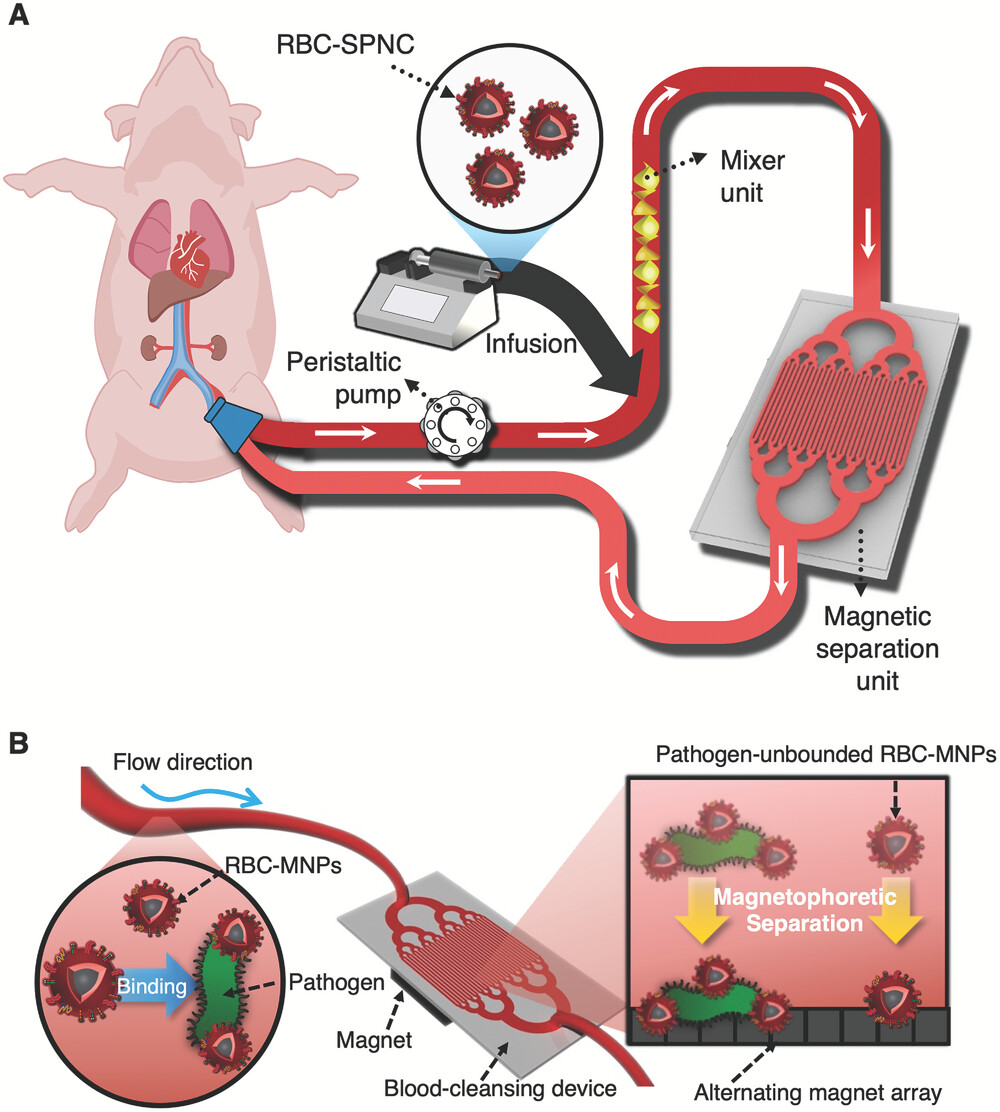
Figure 1. Schematic image, showing the magnetic extracorporeal blood cleansing system utilizes superparamagnetic nanoclusters (SPNCs) enveloped by nanovesicles derived from red blood cells (RBC-SPNCs).
“Our study highlights the significant reduction in pathogenic agents in both the blood and major organs, as well as amelioration of hemodynamic and hematological parameters, followed by the preservation and restoration of major organ functions,” noted Professor Kang. “Furthermore, it was possible to effectively remove a broad range of pathogens and inflammatory agents in the blood and major organs without prior diagnosis, leading to a breakthrough treatment effect.”
Sepsis is a systemic abnormal inflammatory response to infection, which causes the immune system to attack tissues and leads to inflammation and potential organ damage. However, there are currently no effective pharmacological treatments for sepsis until now.
In a previous study conducted in 2022, Professor Kang and his research team developed a similar technology using scavenger MNPs enveloped by nanovesicles derived from blood cells (MNVs), which magnetically eradicate an extreme range of pathogens in an extracorporeal circuit. While their recent report on the potential clinical utility of RBC-coated MNPs for cleansing blood in an extracorporeal circuit in rodents showed promise, it was unsuitable for clinical extracorporeal applications due to its insufficient pathogen capturing and magnetic properties that did not enable efficient magnetic removal of pathogens at flow rates over several liters per hour.
To address this, the team conducted theoretical calculations to determine the optimal magnetic susceptibility and size uniformity of the MNPs necessary to cleanse the blood of sepsis patients within one hour. Through the development of a new hydrothermal synthesis method, they were able to synthesize SPNCs with an improved magnetic susceptibility and size uniformity.
They further validated the clinical utility of their SPNC-based blood cleansing method by treating lethally infected bacteremic model swine. It has also been confirmed that their extracorporeal device can promptly capture and magnetically separate a wide range of pathogens at high blood flow rates (>6 L h−1) in a swine sepsis model.
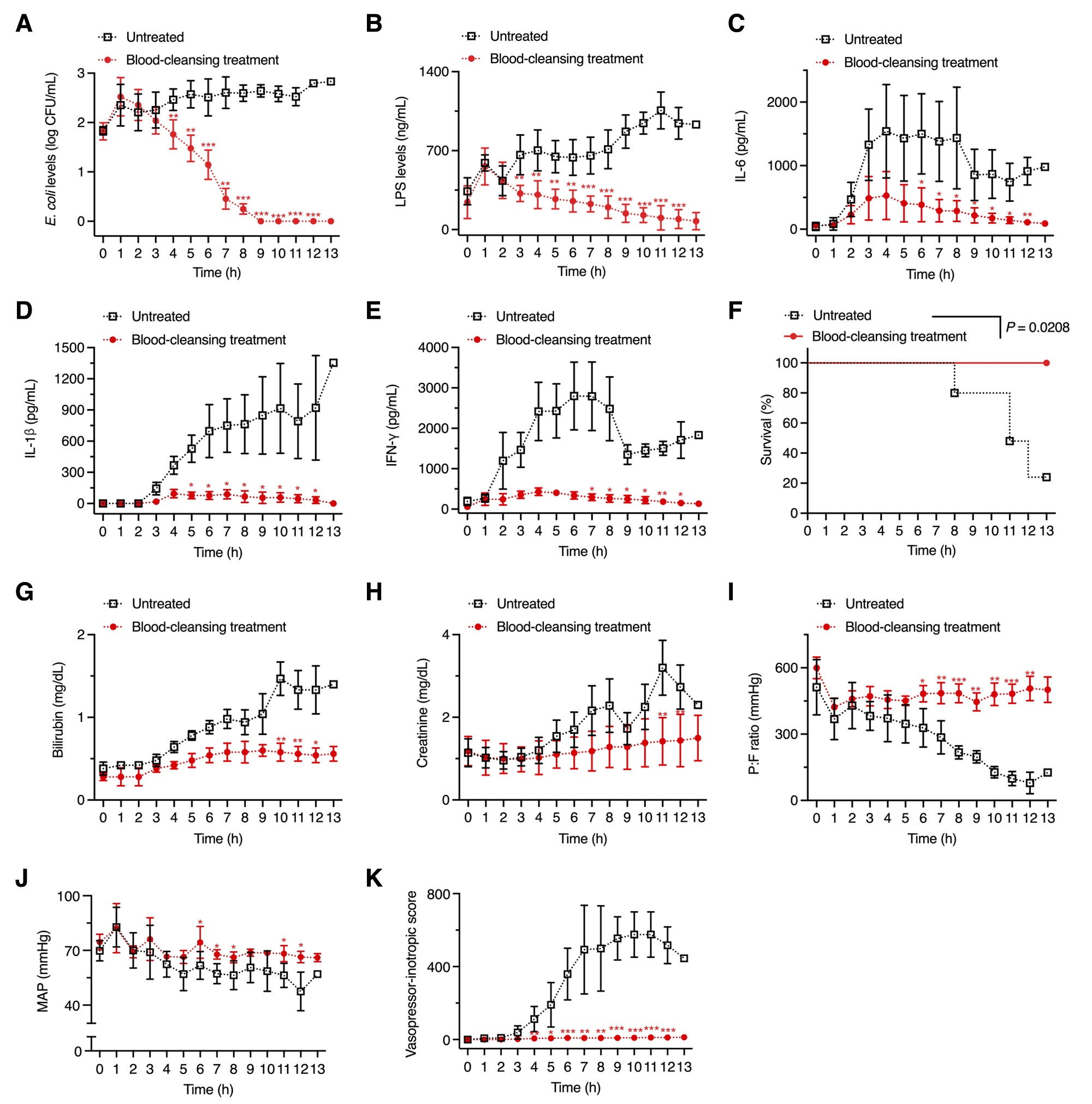
Figure 2. Above shows graphs, depicting the extracorporeal RBC-SPNC blood cleansing treatment which reduces inflammatory triggers in the ESBL + E. coli-infected swine models.
“Our findings demonstrate that the RBC-SPNC-based blood cleansing method improved hemodynamics, attenuated the inflammatory cytokine response, maintained organ function, and increased survival rates,” noted the research team. “We, thus, believe that this treatment could be beneficial regardless of the antibiotics initially used for sepsis treatment.”
Professor Kang explained their plans to validate and introduce medical devices to enable the use of this technology in healthcare. “We aim to develop novel approaches for the treatment of infectious diseases to combat new and mutated pathogens without the need for prior diagnosis, as part of a health security strategy.” He further noted, “Our findings suggest the possible use of RBC-SPNCs for extracorporeal therapy of severe sepsis in large animals and potentially in humans.”
The research findings have been selected as a frontispiece in Small Methods, a world-renowned journal published by Wiley and were officially published on May 17, 2024. The study has been participated by Sung Jin Park and Suhyun Kim in the Department of Biomedical Engineering at UNIST, as well as Professor Inwon Park at Seoul National University Bundang Hospital, as the first authors. This work has been supported by the Samsung Research Funding and the Basic Research Laboratory (BRL) research grant from the National Research Foundation (NRF) of Korea, funded by the Ministry of Science and ICT (MSIT). It was also supported in part by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and Korea Dementia Research Center (KDRC) funded by the Ministry of Health & Welfare and POSCO Science Fellowship of the POSCO TJ Park Foundation.
Journal Reference
Sung Jin Park, Inwon Park, Suhyun Kim, et al., “Extracorporeal Blood Treatment Using Functional Magnetic Nanoclusters Mitigates Organ Dysfunction of Sepsis in Swine,” Small Methods, (2024).