Their findings have been published in Nature Communications June 3, 2019.
DNA replication is essential for living organisms to faithfully deliver genetic information from parental cells to daughter cells. Many proteins are assembled on the parental DNA to work as replication machineries. Among them, the proliferating cell nuclear antigen (PCNA) is a key replication protein. This ring-structured molecule encircles chromosomes, thread-like structures where DNA molecules is packed into, during DNA replication. Just like a ring on a string, PCNA-ring is tightly linked to DNA. After its landing on DNA, PCNA recruits other proteins to efficiently copy the parental DNA. PCNA’s stable connection to DNA makes it an essential platform for many replication machineries.
This is, however, not the end of the story. When the DNA synthesis is complete, PCNA should be removed from the DNA to ensure a complete end of its cycling. Otherwise, the replication proteins make genomic DNA unstable that may lead to genetic mutations. How then are replication machineries cleared from DNA after DNA replication? PCNA unloading from DNA clears replication machineries from DNA after DNA replication. Although PCNA unloading is crucial for maintaining genomic stability, it has been remained unclear how PCNA is unloaded during replication termination.
A research team, led by Director Kyungjae Myung, Professor Hajin Kim, and Dr. Sukhyun Kang at the Center for Genomic Integrity within the Institute for Basic Science (IBS) at UNIST, reported that they revealed a novel molecular mechanism for the regulation of PCNA cycling during DNA replication. They found that a specific protein complex called as ATAD5-RFC-Like-Complex (ATAD5-RLC) is a PCNA-unloader. Dr. Myung’s group has established in vitro reconstitution system by which PCNA loading and unloading could be monitored. They proved that ATAD5-RLC opens PCNA ring to be removed from DNA as a bona-fide PCNA unloader.
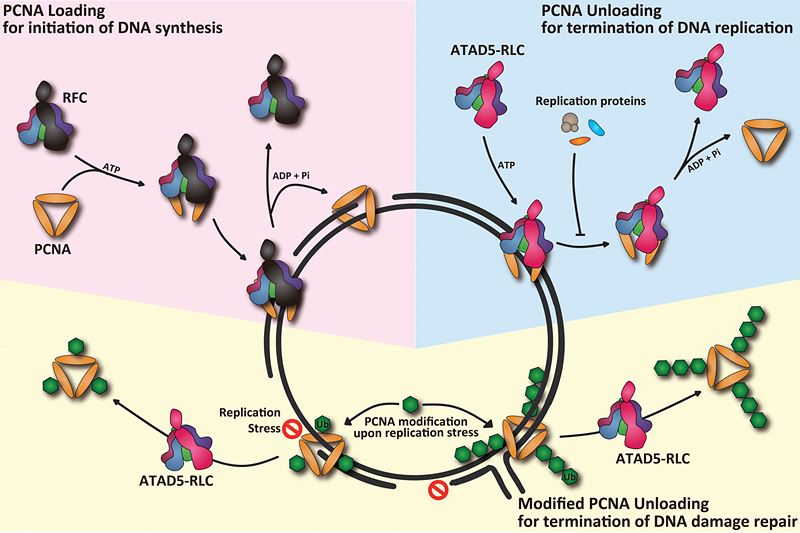
▲ Figure 1. PCNA Cycling during DNA replication.PCNA loading by RFC marks initiation of DNA synthesis. ATAD5-RLC functions as a universal PCNA sweeper that is essential for the termination of DNA replication and repair.
These findings, in fact come from their previous studies. Another protein complex, the Replication-Factor-C (RFC) is known to open PCNA-ring and loads it onto linear DNA molecule before DNA synthesis. Until now, scientists in the DNA replication field believed RFC also function to unload PCNA since bacterial homolog of RFC does both loading and unloading reaction. Dr. Myung’s group suggested that ATAD5-RLC, having a similar structure to RFC, could be a candidate for a PCNA-unloader. This study proved biochemically that ATAD5-RLC is a bona-fide PCNA unloader. The scientists also revealed that ATAD5-RLC could unload modified PCNA, too. PCNA is modified upon replication stress to deal with DNA damages. These findings highlight the role of ATAD5-RLC as a universal sweeper of PCNA for the termination during DNA replication and repair.
The scientists also identified key motifs in ATAD5 that make the protein complex as a PCNA unloader. They revealed the mechanistic differences between PCNA loading and unloading processes. The scientists found a point mutation in a key motif in ATAD5 from melanoma patients, too. Dr. Sukhyun Kang, one of the corresponding authors of this study says, “It was crucial to functionally dissect ATAD5 and purify active ATAD5-RLC. Establishment of in vitro reconstitution system allowed us mechanistic studies for PCNA unloading.”
This study provides complete understanding of PCNA cycling on replicating DNA. It has been controversial which complex is responsible for the unloading of PCNA after DNA replication and repair. The scientists show that two structurally related complexes play distinct roles in PCNA-DNA association. RFC loads PCNA to initiate DNA synthesis and ATAD5-RLC unloads PCNA to terminate DNA replication/repair. Mechanistic difference between loading and unloading of PCNA revealed by this study gives us conceptual advance to understand DNA replication. Since PCNA unloading is closely linked to chromatin assembly, this study will be the basis of future studies for the relationship between replication termination and maintenance of epigenetic information.

Director Kyungjae Myung (School of Life Sciences) of the IBS Center for Genomic Integrity (CGI) at UNIST. l Image Credit:
Director Kyungjae Myung of IBS Center for Genomic Integrity (CGI) believes that this study is a major step toward understanding the regulatory mechanism for replication termination. He noted, “Since uncontrolled disassembly of replication machineries causes genomic instability that may result in cellular transformation, our study will be beneficial to develop strategies for cancer treatment.”
The findings of this research have been published in the online version of the journal, Nature Communications (IF: 12.35) on June 3, 2019.
Myung, Kyung Jae
Distinguished Professor, School of Life Sciences, UNIST
IBS Center for Genomic Integrity
Dahee Carol Kim
IBS Communications Team
T: +82-42-878-8133
E:clitie620@ibs.re.kr
Story Source
Materials provided by Institute of Basic Science.
Notes for Editors
The online version of the original article can be found HERE.
Journal Reference
Mi-Sun Kang et al., “Regulation of PCNA cycling on replicating DNA by RFC and RFC-like complexes,” Nature Communications, (2019). DOI: 10.1038/s41467-019-10376-w

