The spinal cord is a bundle of nerves inside the spine that gives your body structure and support. Spinal cord injuries (SCIs) tend to be devastating and most are permanent. Recent research has shown that motor neuron obtained from skin cells could serve as potential treatments for spinal cord injuries, and thus has received considerable research attention. With this, a new door has been opened for treating not only spinal cord injuries, caused by workplace accidents and car crashes, but also Lou Gehrig’s disease, known as amyotrophic lateral sclerosis or ALS.
A research team, led by Professor Jeong Beom Kim and his research team in the School of Life Sciences at UNIST demonstrated that human fibroblasts can be converted into induced motor neurons (iMNs) by sequentially inducing two transcription factors, POU5F1(OCT4) and LHX3. The research team further investigated the therapeutic effects of iMNs for treating traumatic spinal cord injury using rodent spinal cord injury model. Their findings indicate that the sequential induction of two transcription factors is essential for generating self-renewing iMNICs more efficiently. This method not only ensures large-scale production of pure iMNs, but also facilitates the feasibility of iMNs for SCI treatment.
The spinal cord is responsible for transmitting signals from the brain to the rest of the body, and vice versa. Along with motor and sensory deficits, damage to the spinal cord can cause long-term complications, including limited mobility. Although there are many treatment options available for people with SCI, most of them have adverse side effects that impact therapy. And this is why stem cell (SC) therapies to restore functions of damaged tissues are attracting attention, recently. Among those cells constituting the spinal cord, motor neurons that involved in the regulation of muscle function have emerged as a promising candidate for the stem cell-based therapy for SCIs. Despite these encouraging advances, ethical issue of embryonic stem cells (ESCs) and tumorigenic potential of induced pluripotent stem cells (iPSCs) have impeded their translations into clinical trials.
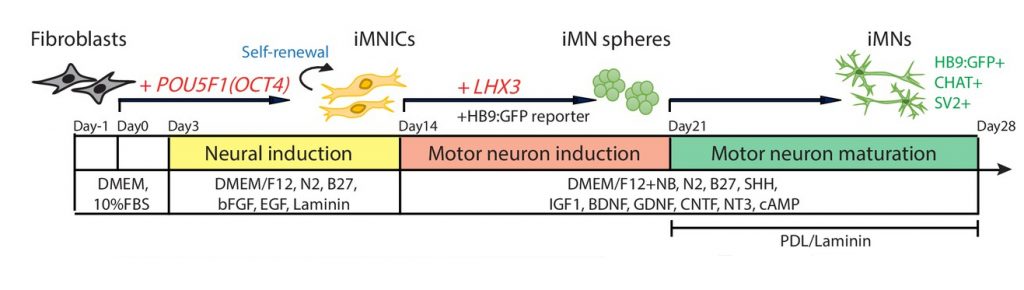
The experimental scheme for the generation of induced motor neurons (iMNs) from human fibroblasts via sequential transduction of two transcription factors. | Image Credit: Professor Jeong Beom Kim
To overcome these limitations, Professor Kim and his research team established an advanced direct conversion strategy to generate iMNs from human fibroblasts in large-scale with high purity, thereby providing a cell source for the treatment of SCI. These iMNs possessed spinal cord motor neuronal identity and exhibit hallmarks of spinal MNs, such as neuromuscular junction formation capacity and electrophysiological properties in vitro. Importantly, their findings also show that transplantation of iMNs improved locomotor function in rodent SCI model without tumor formation. According to the research team, “This proof-of-concept study shows that our functional iMNs can be employed to cell-based therapy as an autologous cell source.” Through this, they resolved the problem of immune rejection, and thus reduce the risk of cancer.
“In the study, we succeeded in generating iMNs from human fibroblasts by overexpressing POU5F1(OCT4) and LHX3,” says Hyunah Lee (Combined MS/Ph.D program of Life Sciences, UNIST), the first author of the study.
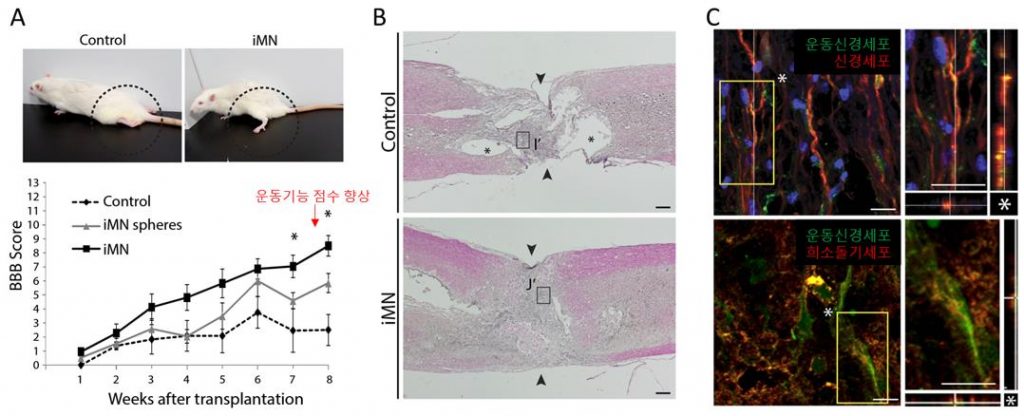
Therapeutic effects of iMNs in rat spinal cord injury model in vivo. (A) The position of hindlimbs in control rat and iMN-transplanted rat after 8 weeks of transplantation. (B) C staining analysis of spinal cords after 8 weeks of transplantation (I; Control, J; iMN-transplanted). l Image Credit: Professor Jeong Beom Kim
The research team found that iMNs exhibited typical characteristics of MNs on molecular level, electrophysiological activity, synaptic functionality, in vivo engraftment capacity and therapeutic effects. The research team noted that their strategy enables large-scale production of pure iMNs and facilitates the feasibility of iMNs for SCI treatment. A sufficient numbers of cells are often required for clinical trials, yet the conventional direct conversion technique is restricted to produce a limited number of cells. On the other hand, the new technique can facilitate the generation of iMNs on large-scale because of the transient acquisition of self-renewing iMN-intermediate cell stage, which is distinct from neural progenitor state. Experimental studies further confirmed that transplantation of iMNs showed therapeutic effects, promoting locomotor functional recovery after 8 weeks in rodent SCI model.
Functional recovery after iMN transplantation in rat SCI model. | Video Credit: Professor Jeong Beom Kim
“Although further investigation on mechanism responsible for cell fate conversion may be needed, our strategy is a safer and simpler methodology that may provide new insights to develop personalized stem cell therapy and drug screening for MN diseases or spinal cord disorders,” says Professor Kim. “If combined with SuPine Patch, an adhesive hydrogel patches with the purpose of regenerating the damaged spinal cords, its therapeutic effects will be maximized.” He adds, “As the incidence of spinal cord injury is high due to industrial accidents, synergistic effects with public hospitals specializing in industrial accidents scheduled to be built in Ulsan should be expected.”
This study has been jointly carried out with Professor Kim’s startup company, SuPine Therapeutics Inc. with the support of the Ministry of SMEs and Startups (MSS). The findings of this research have been published in the 2020 June issue of the online edition of eLife, a renowned academic journal of the European Molecular Biology Organizationl (EMBO).
Journal Reference
Hyunah Lee, Hye Yeong Lee, Byeong Eun Lee, et al., “Sequentially induced motor neurons from human fibroblasts facilitate locomotor recovery in a rodent spinal cord injury model,” eLife, (2020).
