Their findings have been published in the January 2023 issue of Acta Biomaterialia.
A research team, led by Professor Tae-Eun Park in the Department of Biomedical Engineering at UNIST has reported a microphysiological system that emulates the pathophysiological characteristics of human white adipose tissue (WAT). The research team anticipates that their findings may enhance the understanding of obesity-associated disorders and be used to investigate their underlying molecular mechanisms to develop pharmacologic treatment strategies.
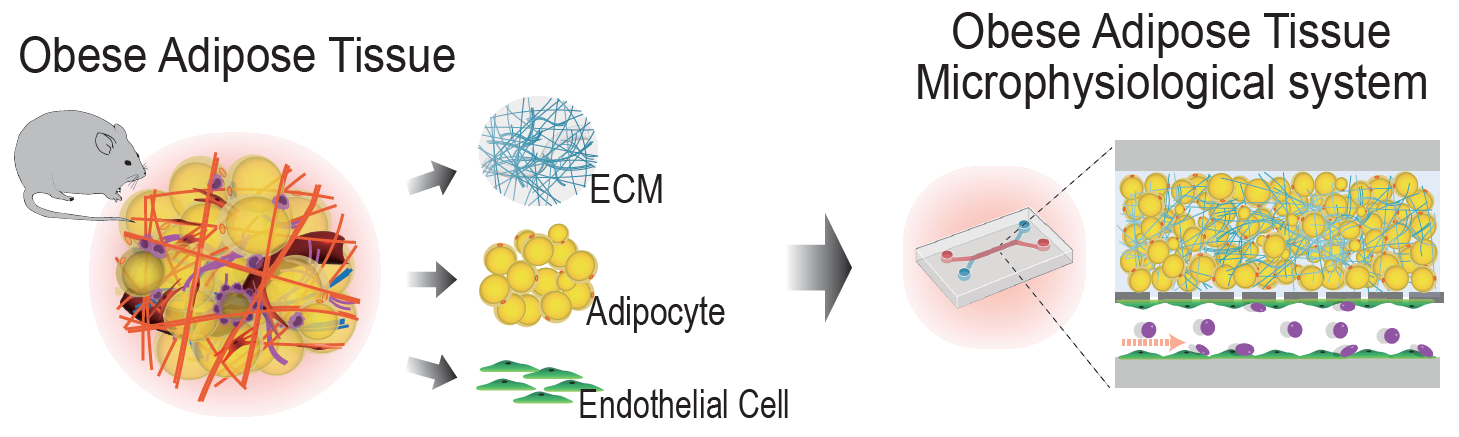
Adipose tissue is a specialized connective tissue mainly composed of fat cells, known as adipocytes. Based on their origin, location, and function, these tissues are categorized into three different cell types—white, brown, and beige adipocytes. Among those,white adipose tissue (WAT) is the predominant type of fat in body. The main functions of WAT are storing energy, insulating body from extreme temperatures, cushioning vital organs, as well as secreting hornones and biological factors.
Despite their obvious advantages, too much white fat may lead to obesity, which is the number one cause of death worldwide. Furthermore, the massive expansion and remodeling of AT during obesity significantly contribute to vascular dysfunction, chronic inflammation, and systemic metabolic disorders, noted the research team. However, with the existing methods, there has been limited understanding of the pathophysiological mechanisms of obesity due to the lack of in vitro models mirroring AT dysfunction in obesity.
In this study, the research team reported the AT microphysiological system (MPS), which recapitulates obesity and normal conditions and yields cell- and AT dECM-derived signals, thereby allowing accurate comparative in vitro analyses. Using the AT MPS, they successfully modeled reprogrammed AT in obesity conditions, including inflammation-induced AT vascular dysfunction, the recruitment of immune cells, and higher cancer cell metastasis, which are observed in obese individuals.
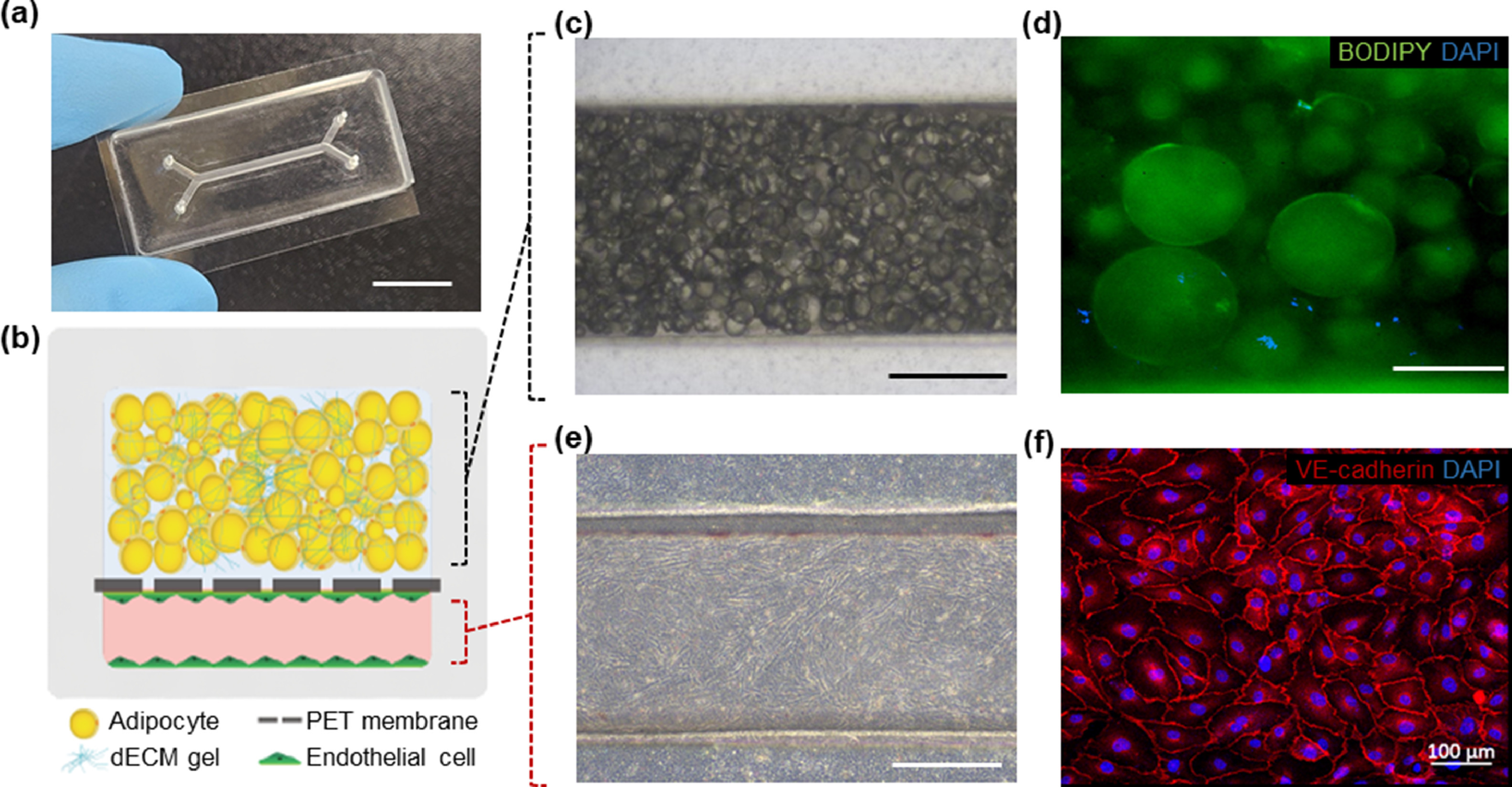
“Our approach offers a perfusable vascular compartment interfaced with a 3D adipocyte construct in a microfluidic system; thus, obesity-associated systemic metabolic disorders may be further modeled by linking with other MPSs, such as the liver and pancreas,” noted the research team. “The unique AT MPS described here may enhance the understanding of obesity-associated disorders and be used to investigate their underlying molecular mechanisms to develop pharmacologic treatment strategies.”
Their findings have been published in the January 2023 issue of Acta Biomaterialia.
Journal Reference
Heejeong Yoon, Jeong Kon Seo, Tae-Eun Park, “Microphysiological system recapitulating the pathophysiology of adipose tissue in obesity,” Acta Biomater., (2023).
UNIST News Center site: https://news.unist.ac.kr/microphysiological-system-recapitulating-the-pathophysiology-of-adipose-tissue-in-obesity/